Gas analysis method name Disadvantage UV gas analysis Poor selectivity, there is a strong inter-air interference; The light source generally has a short life span, the control part is complex, and there is interference with other electrical parts within the instrument. Electrochemical gas analysis Poor stability, poor selectivity, and large flow and ambient temperatures. Easily affected by other gases in the background gas, easy to poisoning failure. On-line chromatography Poor repeatability, poor stability, carrier gas, and troublesome use. Laser gas analysis The light source is expensive, and it has high requirements on the environment and is easily damaged. The semiconductor laser light source is mainly used as a near-infrared light source, and its gas absorption sensitivity is low. Gas name Molecular Formula Infrared feature absorption band range (μm) Analyzer commonly used wavelength (μm) Carbon monoxide CO 4.5 to 4.7 4.66 carbon dioxide CO2 2.75 to 2.8 4.26 to 4.3 14.25 to 14.5 4.27 Methane CH4 3.25 to 3.4 7.4 to 7.9 3.33 Sulfur dioxide SO2 4.0 to 4.17 7.25 to 7.5 7.3 Dioctyl Phthalate,Dop Dioctyl Phthalate,Plastic Industry Plasticizer,Common Plasticizers Shandong Langhui Petrochemicals CO.,LTD , https://www.langhuiplasticizer.com
1. Introduction The spectral absorption method shows that many gas molecules have characteristic absorption in the infrared band; according to Lambert-Beer law, the characteristic absorption intensity is directly proportional to the gas concentration. The infrared gas analyzer designed on the basis of this principle can be used to analyze the concentration of one or several kinds of gas components to be measured in a mixed gas. It is a very important and very classic gas analyzer [1, 2]. Based on the infrared absorption spectrum of gases, the non-monomeric polar gas molecules have a fundamental frequency absorption spectrum of the molecular vibrational energy level in the mid-infrared (2.5 to 25 μm) wavelength band. Therefore, the infrared gas analyzer has high sensitivity and can be used for both applications. Constant analysis can also be used for microanalysis; with good selectivity, it can be achieved that the background gas has almost no effect on the measurement analysis. The well-designed infrared gas analyzer has a good stability and can be used for continuous analysis of gas concentrations and is suitable for on-line measurement.
Compared to other principles of gas analyzers, infrared gas analyzers have significant advantages. Table 1 lists the disadvantages of the four common gas analysis methods. Infrared gas analysis instruments do not have these deficiencies and are more suitable for on-line gas analysis.
Infrared gas analyzer main application areas:
â—‡ Oil, chemical industry, power plants, metallurgical coke and other industrial process controls â—‡ Atmospheric and pollution source emission monitoring and other environmental protection areas â—‡ Restaurants, large conference centers and other public places of air monitoring â—‡ Agriculture, health care and scientific research and other fields such as: (1) The alcoholization tower inlet (out) port of ammonia synthesis process analyzes CO and CO2 with infrared gas analyzer; (2) Decarbonization section of methanol production process, analyzes CO and CO2 with infrared gas analyzer; (3) Environmental emission monitoring, with Infrared gas analyzer analyzes SO2 and NOx.
2. Measurement principle of the infrared gas analyzer The absorption of the mid-infrared light by the measured gas is the basis of the analysis gas of the infrared gas analyzer, and the absorption rule conforms to the Lambert-Beer law.
2.1 Absorption Spectroscopy When a molecule absorbs electromagnetic radiation energy from the outside, electrons, atoms, and molecules are excited and will move from lower energy levels to higher energy levels. The difference in energy before and after the transition is:
E2-E1=hv
Where E2, E1—respectively represent the energy of higher and lower energy levels (energy levels before and after the transition); v—the frequency of the radiation; h—Planck constant, 4.136×10-15 eV·s.
When the energy E of a certain wavelength of electromagnetic radiation is exactly equal to the difference E2-E1 of the energy of a certain two energy levels, it will be absorbed by a certain kind of particle and generate a corresponding energy level transition. The wavelength and frequency of this electromagnetic radiation is called a certain The characteristic absorption wavelength and characteristic absorption frequency of the species.
The fundamental frequency of the vibrational energy level is in the mid-infrared waveband, and the near-infrared waveband is mainly the frequency multiplication and total frequency absorption of various group vibrations. Mid infrared absorption ability, high sensitivity; near infrared absorption weak, low sensitivity.
The absorption spectrum of gas is an absorption band composed of many narrow absorption lines, and can be developed into independent absorption peaks with high-precision spectrometer detection.
Each gas has its own corresponding absorption wavelength, and Table 2 is the characteristic absorption wavelength of common gases.
2.2 Lambert-Beer's Law When the infrared wavelength matches the measured gas absorption line, infrared energy is absorbed. The attenuation of the intensity of infrared light after it passes through the measured gas satisfies Lambert-Beer's law: ![]() (1)
(1) ![]() (2)
(2)
Where L0 and LV represent the intensity of light at an infrared frequency of γ and after passing through a pressure P, a concentration X, and an optical path L, respectively. S(T) indicates the line intensity of the gas absorption line, and the linear function g ( V-v0) Characterize the shape of the absorption line. When the gas absorption is small (absorption rate is low, concentration is low, or the optical path is short), Equation (2) can be used to approximate the gas absorption. These relationships indicate that the greater the gas concentration, the greater the attenuation of light. Therefore, the gas concentration can be measured by measuring the attenuation of infrared light by the gas.
In order to ensure a linear relationship between the readings, when the concentration of the component to be measured is large, the measuring chamber of the analyzer is short, the shortest is 0.3mm; when the concentration is low, the measuring chamber is long, and the longest is >200mm. The remaining light energy after absorption was detected with an infrared detector.
3. Basic structure of infrared gas analyzer The infrared gas analyzer consists of an optical component and a measurement circuit. The structure of the measurement circuit is determined by the optical components and system functions. Optical components are usually made up of infrared radiation sources, gas chambers through sample gas, infrared detectors, etc. These are usually called three major infrared components.
3.1 Infrared radiation source On-line infrared gas analyzers use broad-spectrum (broad-spectrum) light sources. The spectral coverage wavelength of a broad-spectrum light source is from 1 μm to 15 to 20 μm, and the usual range is 2-12 μm. Broad spectrum light sources typically have a band width of a few microns, such as 2-5 μm is one of them.
(1) Continuous light source emits continuous light energy. The motor-driven slicer modulates light to produce infrared radiation of a specific frequency.
An analyzer using a synchronous motor as a motor for cutting requires that the power supply frequency be within a narrow range, such as 50±0.5 Hz, beyond the specified range, which will cause power frequency error.
(2) The light energy emitted from the intermittent light source changes over time, such as a pulsed light source. By controlling the frequency of the electrical signal (voltage or current) of the input light source, infrared radiation of a specific frequency can be generated.
3.2 Infrared instruments for chamber extraction measurements require gas chambers, while in situ and open infrared gas analyzers may not require gas chambers. The gas chamber of the dual optical path analyzer is divided into a measurement chamber and a reference chamber. The measurement chamber continuously passes the sample gas to be measured. The reference chamber is completely sealed and filled with neutral gas (mostly N2). The air chamber of the single-optical path analyzer has only a measuring chamber and no reference chamber.
3.3 Infrared detector The detector of the infrared gas analyzer is used to detect the infrared light energy passing through the air chamber. The detector is divided into two types: pneumatic detector and solid detector. Pneumatic detectors mainly include thin-film micro-phonic detectors and micro-flow detectors; solid-state detectors mainly include photoconductive detectors, pyroelectric detectors, and thermopile detectors.
4. Development of on-line infrared gas analyzer On-line infrared gas analyzer has been widely used in industrial processes as far back as the 1970s, when the analyzers were analog instruments. Subsection linearization design based on analog components can not completely solve the nonlinear problem of infrared analysis (Lambert-Beer's law indicates that infrared analysis is non-linear), the analyzer pointer display, does not have a communication function, often only has a current loop output function .
In the late 1980s and early 1990s, digital gas technology upgrades took place in infrared gas analyzers. After the signal is amplified and filtered, it is sent to the microprocessor, and the analog to digital conversion, linearization, concentration analysis, temperature compensation, pressure compensation, and output display are performed by the micro-processing. The linearization is achieved by piecewise linear or other software algorithms, which is a good solution to the problem. Linear problem. The compensation of ambient temperature and atmospheric pressure is also easy to implement, reducing the impact of the external environment on the analysis process. The liquid crystal display and menu operation realize a good man-machine interface, provide rich display information, and the operation is simple and convenient. The digital communication function facilitates system integration. Digitalization, intelligence, and networking add powerful features to the analyzer. It is the trend of future analyzer development.
There are five types of in-line infrared gas analyzers commonly used: thin-film micro-infrared gas analyzers, micro-flow infrared gas analyzers; gas filter-related infrared gas analyzers, semiconductor infrared gas analyzers, and Fourier infrared gas analyzers. Their principle structure, performance characteristics and major manufacturers at home and abroad are separately elaborated. The content of the principle structure is derived from the reference [1,3-8].
4.1 Thin film micro-phonic infrared gas analyzer The detector of the thin film micro-phonic infrared gas analyzer is composed of titanium metal film movable and fixed electrodes. When the gas pressure in the receiving chamber changes due to the influence of infrared radiation energy, the capacitive film is pushed. Relative to the quiescent movement, the concentration of the measured component is converted into the capacitance change. Thin-film micro-infrared gas analyzers are widely used in online gas analysis, accounting for about 50% of online infrared analyzers.
4.1.1 Principle Structure The basic structure of the QGS-08B thin-film micro-infrared gas analyzer of Beihain Mechak Company is shown in Figure 1. 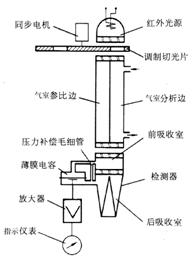
The instrument uses a single light source and a thin film capacitance detector. The measurement chamber and the reference chamber use a "single cylinder spacer" type structure. The receiver chamber is of the series type, with front and rear chambers separated by a wafer. open.
A detector of the film capacitance is mounted on the side of the detector chamber in the two receiving chambers. The two beams of light passing through the reference chamber and the measuring chamber are alternately injected into the front and back absorbers of the detector. The shorter front chamber is filled with the gas to be measured, where the absorption of radiation mainly occurs in the center of the infrared spectrum band, and the longer chamber is also filled with the measured gas. Since the rear chamber adopts a light cone structure, it absorbs. Edge radiation on both sides of the band.
When the measuring chamber passes through the mixed gas (zero point gas) containing no component to be measured, it does not absorb the characteristic wavelength of the component to be measured, and the infrared radiation is absorbed by the components to be measured in the front and rear receiving chambers. The gas is heated, the pressure rises, the pressure on both sides of the capacitor film in the detector is equal, and the geometry of the receiving chamber and the concentration of the charged gas are designed according to the above principles. When the measurement chamber is fed with the mixed gas containing the component to be measured, because the component to be measured has previously absorbed a portion of the infrared radiation in the measurement chamber, the intensity of radiation incident on the detector is reduced. This change in radiation intensity occurs mainly in the center of the band, mainly affecting the absorption energy of the anterior chamber and reducing the absorption energy of the anterior chamber. The infrared radiation absorbed by the components to be measured heats the gas in the front and rear chambers to increase the pressure, but the energy balance has been destroyed. Therefore, the pressures in the front and rear chambers are not equal, resulting in a pressure difference. This pressure difference makes The position of the capacitor diaphragm changes, thereby changing the capacitance of the capacitor, because the radiation source has been modulated, so the amount of change in capacitance is converted into an alternating current signal by the electrical component, and the concentration of the component to be measured is obtained after the amplification.
4.1.2 Performance Characteristics (1) It has good stability and is very suitable for online use.
(2) High sensitivity, which can not only analyze the constant gas, but also analyze the trace gas. It is applicable to a wide measuring range. For example, the trace range of the analyzer is 0-10×10-6CO2, 0-30×10-6CO.
(3) It has strong anti-background gas interference ability, and only the gas corresponding to gas absorption in the detector has sensitivity.
(4) The influence of ambient temperature is small. The change of the ambient temperature has a certain influence on the gas absorption and the sensitivity of the detector. However, if the temperature of the optical component is constant (45-50° C.) and is compensated by software, the influence error can be controlled within ±1% within the operating temperature range.
(5) When the instrument vibrates violently, it will have some influence on the measurement.
4.1.3 Domestic and Foreign Major Manufacturers (1) QGS-08B of Beijing Beifenmeihaike is an analog microanalyzer; QGS-08D is an analog constant analyzer; QGS-08C is a widescreen LED display intelligent analyzer .
(2) PA200-GXH infrared analyzer of Chuanyi No.9 Factory.
(3) ABB Infrared Analyzer Uras26 (original H&B Infrared Analyzer).
(4) MULTOR infrared module of SICK Infrared Analyzer S700 (formerly McHacker Infrared Analyzer).
4.2 Microflow Infrared Gas Analyzer Microflow detection is a gas measurement method that uses the thermal characteristics of a sensitive element to measure changes in the flow rate of a tiny gas. The sensing element is a Wheatstone bridge consisting of two miniature hot wire resistors NiCd grids and two additional auxiliary resistors. The hot wire resistance is energized and heated to a certain temperature. When gas flows, some heat is taken away so that the hot wire element is cooled, the resistance changes, and the voltage signal is converted into a voltage signal through the bridge.
4.2.1 Principle Structure The schematic structure of the ULTRAMAT23 micro flow infrared gas analyzer from Siemens is shown in Fig. 2. 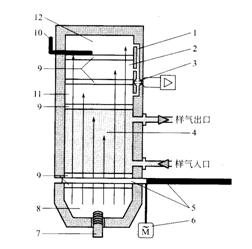
5-cut sheet, 6-cut sheet motor, 7-infrared light source, 8-mirror, 9-optical window,
10-Adjustable Slide, 11 - First Receiving Chamber, 12-Third Receiving Chamber Figure 2 Schematic of the Single Optical Infrared Analyzer Optical System for Microflow Detector
When the infrared light source 7 is heated to 600° C., infrared light is emitted, and the intermittent light beam having a frequency of 25/3 Hz is modulated by the light cutting sheet 5, and the gas chamber 4 enters the gas receiving chamber of the detector.
The receiving chamber consists of a multi-layered tandem chamber filled with the components to be measured. The first layer absorbs the energy in the middle of the infrared radiation band and the second layer absorbs the boundary energy. The two are connected together by the micro-flow sensor 3. . When the light cutting sheet is in the “on†position, the component to be measured filled in the first layer of the receiving chamber 11 absorbs the infrared radiation energy, and is heated to expand, the pressure increases, and the airflow flows through the capillary channel to the second layer of the receiving chamber 2 When the cut sheet is in the "blocking" position, the gas filling of the first chamber is cooled and the pressure is reduced. The gas flow of the second chamber is reversed back to the first chamber by the capillary channel. The light cuts alternately turn off, and the air flows back and forth through the micro-flow sensor to generate an alternating current signal across the detector bridge. The amplitude of the signal is proportional to the gas flow through the sensor, and it is proportional to the concentration of the component to be measured. Inversely.
The micro flow sensor has two nickel grids heated to approximately 120°C. The two nickel grid resistors and the two auxiliary resistors form a Wheatstone bridge. Pulsed air flow repeatedly through the micro-flow sensor, resulting in changes in the resistance of the nickel grid resistor.
The tandem type structure of the receiving chamber is to eliminate the influence of the interference component on the measurement result. In the receiving chamber, in addition to filling the components to be measured, a certain proportion of interference components are filled according to the composition of the measured gas. Interfering components absorb infrared radiation in the first and second layers of the air chamber, and the generated pressure acts in opposite directions and cancel each other out. In the ULTRAMAT23 product, there is also a third-layer receiving chamber 12 whose function is to lengthen the optical path length of the second-layer gas chamber, absorb the infrared radiation edge energy, and adjust the transmissive aperture size of the three-layer gas chamber through the slider. , change the infrared absorption, minimize the influence of an interference component, the effect is equivalent to a dimmable light cone.
4.2.2 Performance Characteristics (1) The dual-channel micro-flow infrared gas analyzer has good stability and can be used online.
(2) High sensitivity to analyze trace gases.
(3) The detector has a simple structure and low cost.
(4) The detector can be used in series and is suitable for simultaneous analysis of multiple components.
(5) The detector is not afraid of vibration and the vibration has no effect on the instrument measurement.
4.2.3 Major Domestic and Foreign Manufacturers (1) The gas flow infrared gas analyzer Gasboard-3000 (new product) of Wuhan Sifang Optoelectronics Co., Ltd. is developed independently by the company and can realize multi-component measurement.
(2) Siemens' ULTRAMAT6 infrared gas analyzer, dual optical path structure, suitable for online use; ULTRAMAT23 infrared gas analyzer, single optical path structure, detector can be connected in series to achieve multi-component measurement.
(3) Fuji Electric's ZRE micro-flow infrared gas analyzer; Yokogawa's IR100 and IR200 micro-flow infrared gas analyzer.
(4) Rosemount's X-Stream Universal Gas Analyzer X2GP.
(5) German BUHLER Company, Beijing Snow Dilong Company and Chengdu Beicheng Company also produce micro-flow infrared gas analyzers and micro-flow detectors adopt Siemens products.
4.3 Gas filter correlation (GFC) Infrared gas analyzer Gas filter correlation detection is a method of deriving the concentration of the analyzed gas by comparing the infrared light transmitted through the reference chamber and the analysis chamber of the relevant wheel.
4.3.1 Principle Structure 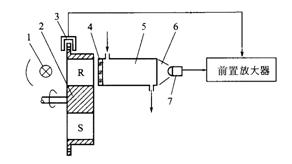
5-Measurement Chamber, 6-Acceptance Chamber, 7-Indium Telluride Detector
Figure 3 is a schematic structural diagram of an infrared analyzer using GFC technology. The filter chamber wheel 2 is equipped with two filter chambers, one of which is an analysis chamber S, which is filled with nitrogen, and the other is a reference chamber R, which is filled with a high concentration of the component gas to be measured. Two kinds of filter chambers are set at intervals. When the filter chamber wheel rotates under the driving of the motor, the analysis chamber and the reference chamber enter the optical system in turn, forming a time-division measurement and reference dual optical path.
When analyzing the air chamber, when S enters the optical path, because S is filled with nitrogen gas and does not absorb infrared light, all the light beams pass through and enter the optical path system to form a measuring light path. When the reference gas chamber R enters the optical path, since R is filled with the component gas to be measured, the characteristic absorption wavelength portion in the infrared light is almost completely absorbed, and the rest enters the optical path system to form the reference optical path.
The infrared light emitted by the light source can be absorbed only by a small part of the component to be measured. In order to increase the selectivity of the instrument, a narrow-band interference filter 4 is added. The central wavelength of the pass band selects the characteristic absorption of the component to be measured. At the peak, only a fraction of the infrared light near the characteristic absorption wavelength passes through the filter into the measurement chamber 5 . The receiving air chamber 6 is a light converging hole, and its function is to converge all the infrared light in the light path on the detecting element.
4.3.2 Performance characteristics (1) Water vapor or other interference components have the same absorption of infrared light transmitted through the two chambers. Therefore, the energy difference received by the detector is only related to the measured gas concentration, and the anti-interference performance is good.
(2) Suitable for small range measurement, can measure 0-30×10-6CO.
(3) The function of the reference gas chamber on the filter chamber wheel is mainly the wavelength reference, eliminating the effect of different infrared absorption wavelengths on the gas. The reference gas chamber does not function as a reference for energy, so it is generally not stable and is strongly affected by the ambient temperature. It is not suitable for online use without reference to the energy.
(4) When measuring a small-range gas, the gas chamber is a multiple-reflection cell, and the cavity has a large volume and a long response time. To reduce the response time, increase the gas flow.
4.3.3 Major Domestic and Foreign Manufacturers (1) Beijing Huayun Analytical Instrument Research Institute (2) Gfx in the Xentra4100 Series of Servomex in the United Kingdom
(2) MODELG FC7000E/7001E/7002E produced by TELEDYNE
4.4 Semiconductor infrared gas analyzer Semiconductor infrared gas analyzer is a very simple gas analyzer.
4.4.1 Principle Structure The basic structure of the QGS-08E semiconductor infrared gas analyzer from Beihaer Machauck is shown in Figure 4. 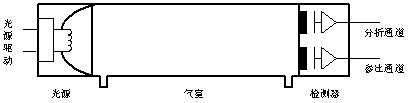
The pulsed infrared light source emits radiation of a specific frequency, which is received by the detector through the air chamber. The detector used in this instrument is a pyroelectric detector with two detection channels: an analytical detection channel and a reference detection channel. When the air chamber is connected to N2, infrared light is not absorbed in the air chamber, and the output signal of the analysis and detection channel is maximized. When the air chamber is connected to the component to be measured, infrared light is absorbed in the air chamber, and the output signal of the analysis and detection channel is reduced. The output signal of the analysis and detection channel changes due to absorption of the component to be measured in the air chamber, and generates an output signal proportional to the concentration of the component to be measured. The output signal of the reference detection channel is not affected by the measured gas and its concentration. It is used to reflect the change of the light intensity of the light source to compensate for the output signal of the analysis and detection channel.
Light source can use electric modulation pulse light source, can also use the traditional motor cut light broad spectrum light source.
Semiconductor infrared detectors include: photo detectors, thermopile detectors, and pyroelectric detectors. The photodetector has a high responsivity and detection rate, but has the characteristic of selective absorption of infrared light. A photodetector can only detect infrared rays in the detectable wavelength range. For example, the detection wavelength range of the indium telluride detector is 2-7um, so it can detect CO and CO2, but it cannot detect NH3 and SO2. Thermopiles are very sensitive to temperature and have a large temperature coefficient of influence and are not suitable as detectors for precision instruments. Pyroelectric detector has the characteristics of wide wavelength response range, high detection accuracy and fast response. The temperature influence coefficient is smaller than thermopile, so it is suitable for gas analysis instruments with high precision measurement.
4.4.2 Performance characteristics (1) The structure is simple, the cost is low, and the production and maintenance are convenient.
(2) Suitable for constant gas analysis.
(3) The gas is distinguished only by the interference filter, the selectivity is not good, and it is easily affected by the interference components in the background gas. However, adding filter air chamber can enhance the anti-interference ability.
(4) Multi-component measurement can be achieved by increasing the number of detector channels.
(5) Semiconductor detectors are greatly affected by temperature. The detector must be thermostatically controlled or software temperature compensated.
4.4.3 Major Domestic and Foreign Manufacturers (1) QGS-08E Infrared Gas Analyzer from Beijing North Branch Maihaike Company.
(2) Sikmahacker's S700 infrared gas analyzer can be equipped with a semiconductor analysis module. The MCS100E is an infrared analyzer using a pyroelectric detector that can analyze at high temperatures and can measure up to 8 gas components.
4.5 Fourier infrared gas analyzer The Fourier infrared gas analyzer uses the interference spectroscopy method and is a spectroscopic instrument.
4.5.1 Principle Structure The optical part of the Fourier Transform Infrared Analyzer consists of a light source, a Michelson interferometer, a sample chamber, and a detector (pyroelectric detector). Its principle structure is shown in Figure 5. 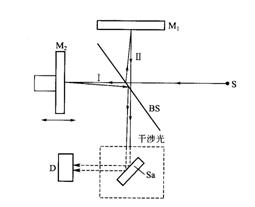
The infrared light emitted by the infrared light source S is collimated into a parallel light beam and enters the interferometer. The interferometer consists of a fixed mirror M1, a movable mirror M2, and a beam splitter BS that forms an angle of 45° with M1 and M2, respectively. The fixed mirror M1 is fixed and the movable mirror M2 can move parallel to the incident light. The beam splitter BS allows half of the incident infrared light to pass through, and the other half is reflected.
After the infrared light emitted by the light source S enters the interferometer, the light beam I passing through the BS is incident on the surface of the movable mirror M2, and the other half is reflected by the BS to the fixed mirror M1 to constitute the light beam II; the light beams I and II are reflected by the passive mirror M2 and the fixed mirror M1 To the BS, it is reflected back to the detector D through the sample chamber Sa. When the two lights I, II reach D, their optical path difference will periodically change with the reciprocating motion of the movable mirror M2, thereby generating an interference phenomenon.
The signal is sent to the data processing system after being sampled by amplifiers, filters, and AD. The Fourier transform transforms the interferogram into an absorption spectrum, and then scans the spectrum library to find a spectrum that matches the absorption spectrum. Through comparison and calculation, the concentration of each component to be measured is obtained.
On-line Fourier gas analyzers have been used for continuous monitoring of emissions from sources of pollution. One of the advantages is that one instrument can measure multiple gas components simultaneously. This feature applies to combustion sources, toxic waste incinerators, and industrial processes. This instrument is best suited for measuring low molecular weight compounds (molecular weight < 50).
4.5.2 Performance characteristics (1) The signal to noise ratio of the spectrum is greatly improved. The number of optical elements used is small, there is no slit and grating spectroscope, so the radiation intensity reaching the detector is high and the signal to noise ratio is large.
(2) Wavenumber measurement accuracy is high.
(3) Strong peak shape resolution.
(4) Scanning speed is fast. Spectral scanning is completed within 1 second.
(5) For a new application object, when adding or changing a measurement component, there is no need to design a new analyzer, just use a new spectrum.
4.5.3 Major Domestic and Foreign Manufacturers (1) ABB's 9200 Fourier Transform Infrared Analyzer.
(2) Fourier infrared analyzer of Sik McHaack.
(3) British ADC Fourier infrared gas analyzer.
5. Engineering application research of on-line infrared gas analyzer Infrared gas analyzer is used on-line in the project. The influence of on-site background gas and working environment conditions on the instrument analysis process must be considered, and these effects must be eliminated or reduced by various methods. Literature [9] proposed the definition, characteristics and origin of generalized interference, and provided a quantitative analysis of the influence of interference and anti-jamming measures, which provided a very effective method for ensuring and improving the accuracy of analyzer measurement. This article analyzes and studies various possible influencing factors in the application of infrared analyzer field engineering, and proposes some feasible solutions to this problem.
5.1 The role of the reference edge According to the Lambert-Beer law, the infrared gas analyzer can measure gas concentration as long as a single chamber or single channel. However, in actual use, the stability of the infrared light source and the detector is not good, and the measurement signal will have a large drift. The light path of the in-line infrared gas analyzer is usually designed as a reference gas chamber or a reference channel. The reference chamber is usually filled with nitrogen or a gas of known concentration. The infrared energy passing through the reference chamber is not related to the absorption of the gas being analyzed. It only reflects the stability of the optical components. Measuring the gas concentration by comparing the energy of the gas cell can greatly increase the stability of the analyzer measurement. The gas chambers of the QGS-08B, QGS-08D, and QGS-08C of the North Branch Mechak Company are single-chamber half-chambers, half of which are analyzer chambers, and the other is a reference chamber. This type of analyzer has very Good stability. The Siemens ULTRAMAT6 is also a dual-optical path structure with a reference gas chamber. The reference channel functions like a reference gas chamber. The infrared wavelength range corresponding to the reference channel usually does not have gas absorption and is only related to the optical component, reflecting the stability of the optical component. Measuring the gas concentration by comparing the analysis channel with the reference channel can effectively improve the analyzer's measurement stability. The detector of the QGS-08E infrared gas analyzer from North Point Mechaco has a reference channel. Sik McHaack's MCS100E also has a reference channel. This type of analyzer has good stability.
Thin-film micro- and micro-flow in-line infrared gas analyzers have a reference gas chamber, and the semiconductor in-line infrared gas analyzer has a reference channel, which has good stability. The Siemens ULTRAMAT23 infrared gas analyzer does not have a reference gas chamber and a reference channel, but it is also sometimes used online, such as in a CEMS system. The ULTRAMAT23 switches the sampling pump periodically, drawing fresh air and zeroing the zero point gas. The long-term stability of the analyzer is achieved by automatic ventilation zeroing. During the automatic zeroing process, the air chamber is filled with zero gas instead of sample gas, and normal measurements cannot be performed. Therefore, this factor needs to be taken into consideration when using in industrial processes.
When the sample gas contains tiny dust particles, the output signal of the reference channel and the analysis channel are attenuated, and the measurement is basically not affected after the offset treatment. However, the reference gas chamber does not have this effect and cannot eliminate this effect.
On-line infrared gas analyzers sometimes have electrical references, for example, through zero input or fixed signal input, measuring the output of the analog channel to improve electrical stability.
5.2 Background Gases Background gases in engineering applications are often very complex and contain other interfering components and water vapor that affect the measurement. The concentrations of interfering components and water vapor are often uncertain and randomly changing. To eliminate or reduce this effect, there are usually the following methods:
(1) The sample gas treatment system removes the interfering components physically or chemically, and reduces the water vapor concentration (dew point) by cooling.
(2) If it is determined that the concentration of the interference component and water vapor is constant, the influence amount can be directly deducted by software.
(3) If the interference component is changed, install a filter chamber in the optical path. The air chamber is filled with high-concentration interference components, and all the infrared energy corresponding to the interference components is absorbed, so that the detector is not affected by the interference components. However, this method reduces the analytical sensitivity.
(4) The optical path is equipped with interference components and water vapor concentration analysis components to detect the interference components and water vapor concentration, and the software deducts the impact of the change in real time.
5.3 The technical indicators and measurement accuracy of the calibration gas online analyzer are limited by the standard gas [10]. If the purity or accuracy of the calibration gas used for the calibration is not sufficient, it will affect the measurement, especially microanalysis.
It is not a gas analyzer that analyzes carbon dioxide. Sometimes it directly draws air as a zero calibration gas. However, in some engineering applications, the air contains components of the gas to be measured and water vapor, which will cause the zero point to be inaccurate and affect the measurement. For gas analyzers that measure carbon dioxide, air is used as a zero-calibration gas for large distances (more than 5%). The same water vapor can cause zero inaccuracies. In the past, the carbon dioxide content in the atmospheric environment was approximately 300×10-6. However, due to atmospheric pollution, the carbon dioxide content in the atmospheric environment is now greater than 400×10-6. If it is a closed cabin, the carbon dioxide content in the air will even be >0.1%.
When microanalysis is needed, the purity and accuracy of the calibration gas need to be paid more attention. For example, after the decarburization of methanol, CO and CO2 gas analyzers with a measuring range of 0-100×10-6 are used. After the zero point and span are calibrated, the process gas is measured, and the reading is often found to be negative. The reason for this phenomenon is that zero-point gas usually uses 99.999% N2, and this zero-point gas contains about 10×10-6 CO and CO2, while the CO and CO2 content in the process gas is particularly low, even lower than that in the zero-point gas. low. The solution to this problem is to add “filtering†to the zero-point gas and remove the CO and CO2 in the zero gas with alkali asbestos and hoggalat.
5.4 Software Processing Online Infrared Gas Analyzer Requirements The Analyzer's software is suitable for online analysis to meet the needs of online engineering applications.
Infrared gas analyzers have zero drift and range drift, and may drift positively or negatively. No restrictions can be placed on the zero point and the end point of the analyzer. The indications below zero and above the end point must be displayed to reflect the drift of the instrument. The same applies to the 4-20mA current loop output. Below 4mA and above 20mA, it must be able to provide output signals.
The resolution of the analyzer should not be too high or too low and should be in the proper range. The general resolution is set to 1/2 - 1/10 of the linear error. If the resolution is too high, the analyzer's indication will frequently jump. If the resolution is too low, the continuity of the analyzer's indication is poor, and it is difficult to guarantee technical indicators such as linear error and drift.
5.5 Temperature The influence of temperature on the infrared gas analyzer is reflected in two aspects, one is the effect of the temperature of the analyzed gas on the measurement; the other is the influence of the ambient temperature on the measurement.
The higher the analyzed gas temperature, the lower the gas sample density and the lower the absorption rate of the gas to the infrared, and thus the lower the measured gas concentration. The thermostatic control of the infrared analyzer can effectively control this influence error.
Ambient temperature affects both optical components (infrared light sources, infrared detectors) and electrical analog channels. Through higher temperature thermostatic control, the use of low temperature drift elements and software compensation can eliminate the effect of ambient temperature on the measurement.
Infrared gas analyzers require that the sun is not directly exposed to the analyzer, to ensure air circulation outside the enclosure and to avoid strong air convection disturbances. The direct sunlight and the flow of large flow velocity will change the thermal balance in the chassis and cause measurement errors.
5.6 Atmospheric pressure The influence of atmospheric pressure on the infrared gas analyzer is mainly reflected in the influence of atmospheric pressure on the gas being analyzed.
The sample gas is directly vented to the infrared gas analyzer. The measurement result is affected by atmospheric pressure. Changes in atmospheric pressure change the density of the sample gas, and the pressure changes the absorptivity of the gas to the infrared, thereby affecting the measurement. Each 1% change in atmospheric pressure causes an error of more than 1%, and for a zero-range infrared gas analyzer, the pressure change has a greater effect. The method to eliminate the pressure effect is to install a pressure measuring element or device to compensate the pressure effect by measuring the atmospheric pressure and reduce the error by an order of magnitude.
When multiple instrument gas lines are measured in series, because the gas pressure is different from that of a single instrument, the measured values ​​will be deviated. The recalibration of the instrument can overcome this effect.
Sample gas flow rate will affect the measurement. For example, the faster the flow rate, the greater the measured value. However, the flow rate does not have a direct effect on the measurement. The effect of the flow rate on the concentration measurement is also not described in the Lambert-Beer law. If the atmospheric pressure remains unchanged, changes in the flow rate will cause the sample gas pressure to change, which in turn will affect the analyzer's measurement.
5.7 电ç£å…¼å®¹å·¥ç¨‹åº”用ä¸ï¼Œé™¤åˆ†æžå™¨æœ¬èº«å¤–,系统内和系统外包括大é‡çš„其它电气设备,电ç£çŽ¯å¢ƒéžå¸¸å¤æ‚,这就è¦æ±‚分æžå™¨çš„è¿è¡Œä¸ä½†ä¸å¯¹å…¶å®ƒè®¾å¤‡é€ æˆå¹²æ‰°ï¼Œä¹Ÿè¦æ±‚分æžå™¨èƒ½æŠµæŠ—ä½å…¶å®ƒéªšæ‰°æºçš„骚扰,具有较强的é²æ£’性。
电ç£éªšæ‰°å½¢æˆç”µç£å¹²æ‰°å¿…须具备三个基本è¦ç´ :(1)电ç£éªšæ‰°æºï¼›ï¼ˆ2)耦åˆé€”径;(3)æ•æ„Ÿè®¾å¤‡ã€‚采用有效的技术手段,抑制骚扰æºã€æ¶ˆé™¤æˆ–å‡å¼±éªšæ‰°çš„耦åˆï¼Œé™ä½Žæ•æ„Ÿè®¾å¤‡å¯¹éªšæ‰°çš„å“åº”æˆ–å¢žåŠ ç”µç£æ•æ„Ÿæ€§ç”µå¹³ã€‚
电ç£å…¼å®¹è®¾è®¡æ—¶ï¼Œé‡‡ç”¨åˆ†å±‚与综åˆè®¾è®¡çš„方法[11]。例如首先分层设计,第一层为有æºå™¨ä»¶çš„选择和å°åˆ·æ¿è®¾è®¡ï¼›ç¬¬äºŒå±‚为接地设计;第三层为å±è”½è®¾è®¡ï¼›ç¬¬å››å±‚为滤波设计。然åŽå†ç»¼åˆè®¾è®¡ã€‚
电路æ¿å¸ƒå±€å¸ƒçº¿ï¼ŒåŒ…括元件的选择都è¦è€ƒè™‘电ç£å…¼å®¹æ€§ã€‚电气走线回路é¢ç§¯éµè¡Œæœ€å°åŒ–原则。
æ£ç¡®å’Œè‰¯å¥½çš„接地å¯ä»¥å‡å°ç›¸äº’间骚扰,å±è”½å’Œæ»¤æ³¢åˆå¯ä»¥é˜»æ–骚扰途径。
抗扰度试验花费很大,但决ä¸å¯ä»¥çœåŽ»ã€‚è¦æ±‚满足GB/T18268-2000附录A[12],性能判æ®ï¼Œå‚ç…§GB/T18268-2000ä¸6.5。
具体试验方法å‚ç…§[13-19]:
1ã€é™ç”µæ”¾ç”µæŠ—扰度:GB/T17262.2-2006ï¼›
2ã€å°„频电ç£åœºè¾å°„抗扰度:GB/T17262.3-2006ï¼›
3ã€ç”µå¿«é€Ÿçž¬å˜è„‰å†²ç¾¤æŠ—扰度:GB/T17262.4-1998ï¼›
4ã€æµªæ¶Œï¼ˆå†²å‡»ï¼‰æŠ—扰度:GB/T17262.5-2008ï¼›
5ã€å°„é¢‘åœºæ„Ÿåº”çš„ä¼ å¯¼éªšæ‰°æŠ—æ‰°åº¦ï¼šGB/T17262.6-2008ï¼›
6ã€å·¥é¢‘ç£åœºæŠ—扰度:GB/T17262.8-2006ï¼›
7ã€ç”µåŽ‹æš‚é™ã€çŸæ—¶ä¸æ–和电压å˜åŒ–的抗扰度:GB/T17262.11-2006。
5.8 多组分分æžå·¥ç¨‹åº”用ä¸ï¼Œç»å¸¸éœ€è¦å¤šä¸ªç»„分åŒæ—¶æµ‹é‡ï¼Œå¤šç»„分分æžå™¨å¯ä»¥ä¸€å°ä»ªå™¨åˆ†æžå¤šä¸ªç»„分,以å‡å°‘仪器数é‡ï¼Œæ–¹ä¾¿ä½¿ç”¨ï¼Œé™ä½Žä½¿ç”¨æˆæœ¬ã€‚
多组分分æžå™¨çš„æž„æˆæ¨¡å¼ä¸»è¦æœ‰ä»¥ä¸‹å‡ ç§ï¼š
(1)多套完整的å•ç»„分光å¦éƒ¨ä»¶å¹¶è”,气室串è”或并è”,构æˆå¤šç»„分åŒæ—¶æµ‹é‡ã€‚
(2)å•å…‰æºã€å•æ°”室和多通é“的检测器构æˆå¤šç»„分åŒæ—¶æˆ–串行测é‡ã€‚åŒæ—¶æµ‹é‡æ—¶éœ€è¦å¤šå¥—模拟放大和滤波部件;串行测é‡ä½¿ç”¨ä¸€å¥—模拟放大和滤波部件,通过模拟开关切æ¢å®žçŽ°å¤šç»„分测é‡ã€‚北分麦哈克公å¸QGS-08F红外气体分æžå™¨å°±æ˜¯è¿™ç±»å¤šç»„分串行测é‡åˆ†æžå™¨ã€‚
(3)å•å…‰æºã€å•æ°”室和多检测器构æˆå¤šç»„分分æžå™¨ã€‚西门åULTRAMAT23å’ŒABB的红外气体分æžå™¨å¯ä»¥å®žçŽ°è¿™ç±»å¤šç»„分气体分æžå™¨ã€‚
(4)å•å…‰æºã€å•æ°”室ã€å•æ£€æµ‹å™¨å’Œåˆ‡å…‰è½®ï¼ˆåˆ‡å…‰ç‰‡ï¼‰æž„æˆå¤šç»„分分æžå™¨ã€‚相关红外ã€è–„膜微音和åŠå¯¼ä½“红外å¯ä»¥é‡‡ç”¨è¿™ç§æ–¹å¼å®žçŽ°å¤šç»„分串行测é‡ã€‚西克麦哈克公å¸S700å’ŒMCS100E就是这类多组分气体分æžå™¨ã€‚
(5)傅立å¶çº¢å¤–气体分æžå™¨ã€‚é…置一套光路,通过分æžçº¢å¤–干涉光谱,å¯ä»¥åŒæ—¶æµ‹é‡è®¸å¤šç§æ°”体组分浓度。
多组分分æžå™¨çš„å„个组分之间å¯èƒ½å˜åœ¨å¹²æ‰°ï¼Œæˆ–å—背景气体干扰影å“。å¯ä»¥é€šè¿‡æµ‹é‡å¹²æ‰°ç»„分浓度和干扰影å“率,软件扣除干扰组分的影å“。例如MCS100E测é‡æ°´è’¸æ°”浓度,从而扣除水蒸气对其它气体测é‡çš„å½±å“。
如果多组分分æžå™¨ä¸åªæœ‰ä¸€ä¸ªæ°”室,就需è¦ç»¼åˆè€ƒè™‘å„个组分浓度和å¸æ”¶çŽ‡ï¼Œé€‰æ‹©åˆé€‚的气室长度。有时还需è¦è°ƒæ•´å¹²æ¶‰æ»¤å…‰ç‰‡çš„é€è¿‡æ³¢é•¿ï¼Œä»¥æ–¹ä¾¿æ°”室的选å–。例如åŒæ—¶åˆ†æžé«˜æµ“度CO2和低浓度CO时,CO2干涉滤光片就需è¦é€‰æ‹©å…·æœ‰è¾ƒä½Žå¸æ”¶çŽ‡çš„波长。
6ã€ç»“æŸè¯ç”±äºŽçµæ•åº¦é«˜ã€ç¨³å®šæ€§å¥½ã€ç®€å•å¯é 的特性,在线红外气体分æžå™¨åœ¨å„工业ã€çŽ¯ä¿åŠç§‘ç ”é¢†åŸŸå¾—åˆ°äº†å¹¿æ³›çš„åº”ç”¨ã€‚æœ¬æ–‡é€šè¿‡å¯¹å‡ ç§å¸¸ç”¨çº¢å¤–气体分æžå™¨çš„原ç†ç»“æž„ã€æ€§èƒ½ç‰¹ç‚¹åŠå›½å†…外主è¦åŽ‚家相关产å“的介ç»ï¼Œé˜è¿°äº†çº¢å¤–气体分æžå™¨çš„å‘展状况。数å—化ã€æ™ºèƒ½åŒ–和网络化给红外气体分æžå™¨å¢žæ·»äº†å¼ºå¤§çš„功能,是未æ¥çº¢å¤–分æžå™¨å‘展的趋势。针对红外气体分æžå™¨åœ¨çº¿åº”用的特点,本文分æžäº†æµ‹é‡è¯¯å·®çš„多ç§å½±å“å› ç´ ï¼Œæ出了能够å‡å°æµ‹é‡è¯¯å·®ã€é€‚åˆåœ¨çº¿åˆ†æžã€å¹¶æ高分æžå™¨çŽ°åœºåº”用能力的若干方法,进而æ高测é‡å‡†ç¡®åº¦ï¼Œæ›´å¥½æ»¡è¶³åœ¨çº¿å·¥ç¨‹åº”用的需è¦ã€‚
Development and Engineering Application of Infrared Gas Analyzer
Abstract: Infrared gas analyzer is a kind of widely used and most representative on-line gas analyzer with high sensitivity and good stability. Common non-elementary gases may be applied. This article briefly describes the measurement principle, basic structure and development trend of infrared gas analyzer. Several types of commonly used infrared analyzers and major domestic and foreign manufacturers' products are introduced. When the actual field is used, the background gas and the working environment are complex and varied. This paper deeply analyzes the complex influencing factors of measurement error, and studies several methods to improve the field application capability of the analyzer to enhance the adaptability and strength of the application of infrared gas analyzer engineering. . On-line analysis of the application and development of engineering technology requires an on-line analyzer, a solid and strong technical foundation.
Table 1 Disadvantages of other four common gas analysis methods
Table 2 Characteristic absorption wavelengths of common gases
Fig.1 Schematic diagram of QGS-08B thin-film micro-infrared gas analyzer
1- capillary gas flow channel, 2-layer receiving chamber, 3-micro flow sensor, 4-measured gas chamber,
1-Light Source, 2-Filter Air Chamber Wheel, 3-Synchronous Signal Generator, 4-Interference Filter,
Fig. 3 Principle of infrared analyzer using GFC technology
Figure 4 Principle structure diagram of QGS-08E infrared analyzer
S-light source, Ml mirror, M2 moving mirror, BS-beam splitter, D-detector, Sa-sample chamber
Fig. 5 Principle of the Fourier infrared gas analyzer