Guangzhou YS Auto Parts Co.,LTD , https://www.upgradebodykit.com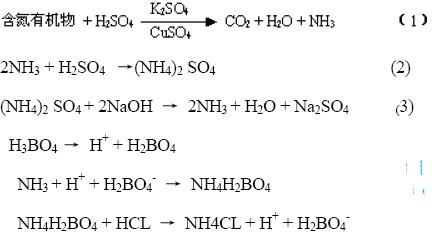
Quantitative determination of crude protein - trace Kjeldahl method
First, the purpose of the experiment
1. Master the Kjeldahl method for determination of protein content.
2. Learn to use Kjeldahl.
Second, the experimental principle
Proteins consist of carbon, hydrogen, oxygen, nitrogen, and a small amount of sulfur. The content of these elements in the protein has a certain proportion of the relationship, including carbon 50 ~ 55%, hydrogen 6 ~ 8%, oxygen 20 ~ 23%, nitrogen 15 ~ 17% and sulfur 0.3 ~ 2.5%. In addition, trace elements such as phosphorus, iron, zinc, copper, and molybdenum are contained in some proteins.
Since nitrogen is a characteristic of proteins that distinguish them from sugars and fats, and the nitrogen content of most proteins is quite similar, it is generally constant at 15 to 17% with an average value of about 16%. Therefore, in the quantitative analysis of proteins, every measurement One gram of nitrogen is equivalent to 6.25 grams of protein. Therefore, as long as the nitrogen content in the biological sample is determined and multiplied by 6.25, the protein content in the sample can be calculated.
The amount of nitrogen in a biological sample can be determined using the following reaction:
Nitrogen-containing organic matter is heated together with concentrated sulfuric acid and is oxidized to carbon dioxide and water, while nitrogen is converted to ammonia. Nitrogen is further reacted with sulfuric acid to produce ammonium sulfate. The process of breaking down macromolecules into small molecules is often called "digestion." In order to accelerate the digestion, it is usually necessary to add potassium sulfate or sodium sulfate to increase the boiling point of the digestive liquid (290°C→400°C), add copper sulfate as a catalyst, and hydrogen peroxide as an oxidant to promote the reaction.
The reactions (1) and (2) are completed in a Kjeldahl flask, and the reaction (3) is carried out in a Kjeldahl distillation unit, which is characterized by the integration of the three parts of a steam generator, a distiller, and a condenser. Because the steam generator is small and saves energy, the instrument is easy to use and has good results. Ammonium sulphate and concentrated alkali can free ammonia, the water vapor will be produced by distillation of ammonia to a certain concentration of boric acid solution, boric acid absorption of ammonia to make the solution in the H + concentration decreased, and then titrated with standard inorganic acid, until the recovery solution Before the original H+ concentration, the total amount of nitrogen in the analyte was finally calculated based on the amount of standard acid used.
Third, instruments and reagents
Instrument: digestive tube or Kjeldahl flask, Kjeldahl distillation apparatus, electric furnace, digestion furnace, 100mL conical flask, 100mL measuring cylinder, watch glass, acid burette, small funnel, glass beads, etc.
Reagent
1. Other protein-containing samples such as serum or ovalbumin
2. Digestion solution: The ratio of 30% hydrogen peroxide, sulfuric acid and water is 3:2:1, and it is prepared when it is used.
3. Catalyst: Copper sulfate (CuSO4 • 5H2O) and potassium sulfate (K2SO4) were ground and mixed in a ratio of 1:3.
4. 50% sodium hydroxide solution
5. 2% boric acid solution
6. Standard hydrochloric acid solution (about 0.01mol/L)
7. Mix indicator (Tian's indicator) mixing indicator is prepared by mixing 50 mL of 0.1% methylene blue ethanol solution with 200 mL of 0.1% methyl red ethanol solution and storing it in a brown bottle. The indicator is purple when acidic, green when alkaline, and the color change is very narrow and sensitive.
Fourth, the experimental steps
(I) Installation of Kjeldahl nitrogen meter
The nitrogen determination instrument consists of a steam generator, a reaction chamber, and a condenser tube. The steam generator includes an electric furnace and a 3 to 5 liter volume flask. Above the reaction chamber there are two small beakers, one for the sample and one for the lye. The sample and the lye can thus be directed into the reaction chamber. The center of the reaction chamber has a long glass tube, with the upper end leading to the outside of the reaction chamber and the lower end near the bottom of the reaction chamber. The bottom of the lower end of the reaction chamber has an opening with a rubber tube and a pipe clamp. This releases the reaction waste. The nitrogen produced by the reaction can pass through the upper end of the reaction chamber through the condenser tube to the collection bottle. The reaction chamber and the condenser are connected by a rubber tube.
When the instrument is installed, the steam generator is vertically fixed on the iron stand and the steam generator, the reaction chamber, and the condenser are connected by a rubber pipe. Rubber hose connections should be in the same horizontal position. The distance between the lower end of the condensing tube and the lab bench is based on the lower collection bottle. After installation, do not move easily to avoid damage to the instrument. To carefully check the entire device for leaks, to ensure the accuracy of the measured results.
(b) Treatment of samples
(1) Solid sample randomly take a certain amount of finely ground sample into a constant-weight weighing bottle and place it in an oven at 105°C for drying for 4 hours. Remove the weighing bottle into a desiccator with forceps to wait until it has fallen. Weigh at room temperature, then continue to dry the sample, dry for 1 hour, weigh once, constant 0 weight.
(2) Serum samples Take human blood (or porcine blood) into a centrifuge tube and place in the freezer overnight. The next day, the blood clot was removed by centrifugation and the clear supernatant was serum. Aspirate 1mL of serum into a 50mL volumetric flask, dilute to volume with distilled water, and mix until ready. If the solution is cloudy, add a small amount of sodium chloride and mix well.
(3) Ovalbumin 2 g Ovalbumin was dissolved in 0.9% NaCl solution and diluted to 100 mL. If there is any insoluble matter, centrifuge the supernatant for use.
(III) Digestion
Take 5 digestive tubes and number them, and add dry samples accurately in tubes 1, 2 and 3 (Note: When adding samples, feed directly into the bottom of the tubes to avoid sticking to the nozzles and necks) and add catalyst. 0.5g, mixed digestive solution 3mL, add the same amount of catalyst and mixed digestion solution (if the sample is liquid, add the same volume of distilled water to the sample) in the 4th, 5th tube, respectively, as a control to determine the reagent Traces of nitrogenous substances that may be contained. After shaking, five digestive tubes were placed on a far-infrared cooking stove in a fume hood for digestion. First boiled with low heat and boiled, soon the material in the digestive canal is blackened and a large amount of foam is produced. Special attention must be paid at this time. Black matter cannot be allowed to rise to the neck of the digestive tract, or it will seriously affect the sample determination results. When the mixture stops bubbling and steam and carbon dioxide are also released evenly, the firepower is appropriately enhanced. During digestion, all samples should be soaked in the digestive fluid. If black particles are found on the bottleneck, the digestion should be carefully shaken and rinsed with digestive fluid. Normal digestion takes 1 to 3 h (longer time is required for samples with higher lysine content). After the digested liquid turns brown, in order to speed up digestion, the digestive canal can be taken out, cooled slightly, and 1 to 2 drops of 30% hydrogen peroxide solution can be added to the bottom digestive solution, and heating is continued for 0.5 hours. After digestion, remove the digestive tube and cool it to room temperature.
(d) Distillation
(1) The instrument's washing equipment should be washed first and then steam. The purpose is to wash away any ammonia that may remain in the condenser. For the instrument in use (instrument under measurement), steam is allowed to pass for 1 to 2 minutes before adding the sample. For instruments that have not been used for a long time, they must be washed with steam to the steam-absorbed boric acid indicator mixture. The color of the agent is acceptable. The washing method is as follows:
Take 2 to 3 100 mL Erlenmeyer flasks, add 10 mL of 2% boric acid, 2 drops of mixing indicator and cover with a watch glass. A steam generator is now being boiled, which contains 2/3 volumes of distilled water acidified with a few drops of sulfuric acid, and 2â„3 volumes of distilled water are also added to the sample cup for water sealing. The clip is closed so that the steam enters the reaction chamber through the cannula in the reaction chamber and escapes from the lower end of the condenser. Place an empty beaker on the lower end of the condenser to withstand agglutination. In this way, steam is used to wash for about 5 minutes. Place a prepared conical flask containing boric acid-indicator under the condensing tube. The position is inclined. The lower part of the condensing tube should be completely immersed in the liquid and continue to be washed with steam for 1 to 2 minutes. Observe that the solution in the Erlenmeyer flask does not substantially change color. If it does not change color, it indicates that the interior of the distiller has been washed clean. Move the conical flask down so that the boric acid surface is about 1 cm away from the condensing nozzle and continue steaming for 1 min. Finally, rinse the outside of the condenser with distilled water. Use the right hand to gently lift the rod in the sample cup.
When the water flows into the reaction chamber, immediately close the clip with your left hand and cover the stopper. Due to the cold shrinkage and pressure drop in the reaction outside layer, the waste liquid in the reaction chamber is automatically pumped into the reaction chamber shell through the reaction chamber intubation, and then 2/3 volume of distilled water is added to the sample cup, so that the waste can be exhausted three times in a row. Liquid and washing liquid. Open the clamp to discharge the waste liquid accumulated in the reaction chamber housing. Close the clamp and let the steam pass through the complete distillation apparatus for 1~3min to perform the next distillation.
(2) Distillation of sample and blank Take 5 100 mL conical flasks, add 2% boric acid 10 mL respectively, mix 2 drops of indicator, the solution is purple-red, cover it with a watch glass.
Transfer the digestive juice from the digestive tube to the sample cup. Rinse the digestive tube with about 2 ml of distilled water. Repeat 3 times. Pour the wash solution into the sample cup, open the rod-shaped glass plug of the sample cup, and place the sample into the reaction chamber. After flushing the sample cup with a small amount of distilled water, it also flows into the reaction chamber, covers the glass plug, and adds about 2/3 volume of distilled water to the sample cup for water sealing. Then place the conical flask containing the boric acid-indicator under the condensation nozzle, open the clip for storing the lower end of the alkaline solution cup, put 10 mL of 40% sodium hydroxide solution in the reaction chamber, and immediately lift up the conical flask to make the condenser tube. The lower mouth is submerged under the liquid surface of the conical flask. After the reaction liquid boils, the boric acid-indicator mixture in the Erlenmeyer flask turns from purple red to green, and starts timing when it is discolored, and is distilled for 3 to 5 minutes. Move the Erlenmeyer flask so that the boric acid liquid surface is separated by about 1 cm. Rinse the outside of the condenser tube with a small amount of distilled water and continue to distill for 1 min. Rinse the tip of the condenser tube with a small amount of distilled water. Remove the Erlenmeyer flask and cover it with a watch glass to be titrated. . Discharge and wash are the same as before. After washing and washing, the next sample can be distilled (each sample must be taken in triplicate for accurate results). After the distillation of the sample and blank digestion solution was completed, titration was performed at the same time.
(v) Titration
After all the distillation is completed, titrate the amount of ammonia collected in each Erlenmeyer flask with 0.001 mol/L standard hydrochloric acid until the boric acid-indicator mixture changes from green to light grape purple. This is the endpoint of the titration. Record the amount of HCl solution consumed. .
V. Calculation
The total nitrogen content of the sample (%) = (A - B) × 0.001 × 14.008 × 100/1000 × C
If the nitrogen content of the sample is only protein (eg serum), then:
The content of protein in the sample (%) = (A-B) × 0.001 × 14 × 6.25 × 100/1000 × C
A- titration sample used hydrochloric acid volume (mL);
B-titration blank used hydrochloric acid volume (mL);
C - The amount of sample weighed (g);
0.001-molar concentration of hydrochloric acid (mol/L);
14-amount of nitrogen atoms;
The 6.25-coefficient (1 mL of 0.001 mol/L hydrochloric acid corresponds to 0.14 mg of nitrogen).
If there are other nitrogenous substances besides the protein in the sample, the determination of the protein content of the sample is more complicated. First, trichloroacetic acid needs to be added to the sample to a final concentration of 5%, and then the nitrogen content in the supernatant of the sample without trichloroacetic acid and the trichloroacetic acid added is determined to obtain non-protein nitrogen. The amount of protein nitrogen was calculated, and the protein content was further calculated.
Protein Nitrogen = Total Nitrogen - Non-Protein Nitrogen
Crude protein content (%) = protein nitrogen × 6.25